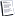
The Carnot Cycle states that wherever a temperature differential exists, energy can be extracted. The Carnot efficiency is the general theorum for converting heat energy to energy capable of doing work. It works over a temperature differential (hot and cold side). The equation is:
Carnot efficiency = 1 - [T(cold) / T(hot)]
Obviously the larger the difference in temperature of the hot and cold side, the more efficient the engine can be. Stirling Engines don't work very well in "low-grade heat" (low temperature differential). Heat is the same energy used to perform work, just in a different form. The quest has always been on to master the conversion of heat to energy that we can currently use. The energy crisis has just sped it up.
Heat, however, likes to move from the hot side to the cold side on its own, by radiation. This is where it gradually spreads out until it heats up the cold side, and reaches equilibrium (same temperature). The Carnot Cycle tries to convert this heat to mechanical energy before this can happen.
Thermoelectric generators also follow the carnot cycle... they require a temperature differential to be present.
|